-
Filter
- Chalk
- Skimmer ACF
- LSS tank "Basic"
- MultiFunctionFilter MF2
- Globuli Filter
- Pellet Filter POC
- Nitrate Filter ADN
- Neutralisierungsfilter NF
- Phosphate Filter PO4
- Fluidized Bed Filter FBF
- Activated Carbon Filter AK
- Zeolite Filter
- Trickling Filter TKF
- Sponge Loop Filter
- Ozone Reactor OZR
- CO2 reactor COR
- Gas filters
- Small Filter
- Empty Filter
- Filter Materials
- Filter Accessories
- UV lamps
- Additives
- Measuring
- Keeping/Breeding
- Water
- Pumps
- Fittings
- Installation
- Info
AquaCare-shop.de Info Encyclopaedia Seawater Chemistry&Physics Barium
Barium has similar chemical properties to calcium and is also incorporated in coral skeletons as witherite during the precipitation process (occlusion). The species-specific barium-calcium ratio in the skeleton increases with increasing barium concentration and decreasing temperature. The ratio of different barium isotopes in the coral skeleton gives an indication of the salinity at which the coral grew.
Biological relevance: dissolved barium is basically toxic relatively low concentrations and is not essential (necessary for life) according to current knowledge. Barium precipitates very quickly to insoluble compounds and thus loses its toxicity. It is not known whether there are redissolution processes in the substrate or reef rock of marine aquariums and thus the bioavailability increases again.
Barium can be introduced into the aquarium through tap water, food, salt mixtures, aquarium cement or through activated carbon and zeolite. - Barium in drinking water causes reverse osmosis membranes to block extremely quickly and should be made harmless by a suitable pre-filter.
The easiest and safest way to reduce too high barium concentrations is to perform a water change. Adsorbers act too unspecifically.
Natural seawater concentration: 2-93 µg/l
Recommendation for seawater aquariums: 10...100 µg/l
ICP measurements do not distinguish between dissolved (toxic) and undissolved (non-toxic) barium.
David W. Lea, Glen T. Shen & Edward A. Boyle 1998: Coralline barium records temporal variability in equatorial Pacific upwelling.
Tianran Chen, Kefu Yu, Shu Li, Tegu Chen, Qi Shi 2011: Anomalous Ba/Ca signals associated with low temperature stresses in Porites corals from Daya Bay, northern South China Sea.
Zhimian Cao1, Xinting Rao1, Yang Yu, Christopher Siebert, Ed C. Hathorne, Bo Liu, Guizhi Wang1, Ergang Lian, Zhibing Wang, Ruifeng Zhang, Lei Gao, Gangjian Wei, Shouye Yang, Minhan Dai1, and Martin Frank 2021: Stable Barium Isotope Dynamics During Estuarine Mixing.
Accessories
| Product | Note | Status | Price | |
|---|---|---|---|---|
|
protects the reverse osmosis unit against barium |
|
95.20 € * | |
|
*
Display accessory details
Prices incl. VAT, plus delivery
|
||||
Browse these categories as well: Chemistry&Physics, Home page


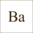
 10" antiscaling filter (prevents lime scale)
10" antiscaling filter (prevents lime scale)