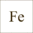-
Filter
- Chalk
- Skimmer ACF
- LSS tank "Basic"
- MultiFunctionFilter MF2
- Globuli Filter
- Pellet Filter POC
- Nitrate Filter ADN
- Neutralisierungsfilter NF
- Phosphate Filter PO4
- Fluidized Bed Filter FBF
- Activated Carbon Filter AK
- Zeolite Filter
- Trickling Filter TKF
- Sponge Loop Filter
- Ozone Reactor OZR
- CO2 reactor COR
- Gas filters
- Small Filter
- Empty Filter
- Filter Materials
- Filter Accessories
- UV lamps
- Additives
- Measuring
- Keeping/Breeding
- Water
- Pumps
- Fittings
- Installation
- Info
AquaCare-shop.de Info Encyclopaedia Seawater Chemistry&Physics Iron
Iron is essential for all organisms and plays a decisive role in blood formation in particular. Only crustaceans use copper as the central atom of the blood pigment. Iron is also required for some enzymes, e.g. in addition to molybdenum, iron is contained in nitrogenase, an important enzyme in nitrogen fixation. Iron is also essential for chlorophyll formation. In teeth of Chaetodontidae, iron provides hardness.
Because iron is so important and it is normally found only in low concentrations in most biotopes (iron limitation), different strategies have been developed to obtain this metal. Anaerobic organisms take up the reduced iron (Fe-II) directly because it is present in the bioavailable form in these biotopes. So-called siderophores are low molecular weight compounds (200...300 D) produced by some aerobic groups of organisms to capture trivalent iron (Fe-III). Phyto-siderophores also allow some plants to more easily obtain the iron they need. The siderophores are excreted, loaded with iron-III in the outside world, and shuttled back into the cell. By reducing the trivalent iron to divalent iron (Fe-II) in the cell, the iron is again released from the siderophore and utilized. Some organisms do not form their own siderophores and simply help themselves to the existing iron-siderophore complexes (xenosiderophores) in the water. Slightly elevated iron concentrations can trigger algal blooms. High iron concentrations can quickly show toxic effects.
Corals deficient in iron show weak colors. Iron deficiency increases the effects of thermal stress in corals (bleaching). Iron deficiency can alter intracellular concentrations of zinc, copper, cobalt, manganese, nickel, molybdenum, vanadium in zooxanthellae (Symbiodiniaceae) and thus affect the entire holobiont coral.
Iron addition especially in combination with higher nitrate levels increases the density with zooxanthellae (brown coloration of the tissue) up to the partial loss of the symbiotic algae. Elevated Fe levels can trigger deficiencies of other trace metals due to an unfavorable ratio, thus indirectly damaging corals. Elevated iron levels may increase respiration of holobiont-associated bacteria and other organisms, leading to oxygen deficiency of the coral. The observed reduction in nitrogen fixation by specialized bacteria affects the nitrogen supply to the holobiont. It is discussed that the higher temperature resilience of Red Sea corals can be explained by a better supply of iron due to regular input of desert dust.
High iron concentrations above a few 10 mg/l can damage sensitive coral larvae.
Iron-III can be deposited in the skellet by corals from tidal areas (river water input) - visible as brown bands in the skeleton. This can be interpreted as a protective device against high iron concentrations.
Before iron is dosed, it is essential to perform ICP-MS analysis. ICP-OES is not sufficient to detect a deficiency. If iron is post-dosed, it should definitely be in the divalent form, as not all organisms can form siderophores and capture the trivalent iron.
Iron sources:
Sea salt, iron additives, feed, plankton, iron-based phosphate adsorbers, lime reactors, defective pumps, steel parts such as screws. In nature, also from river water.
Iron sinks:
Zeolites, water changes, precipitation processes.
Recommendation for seawater aquaria: 1...3 µg/l
Natural seawater concentration: 0.1..62 µg/l (at 35 PSU).
Translated with www.DeepL.com/Translator (free version)
Iron-III can be deposited in the skellet by corals from tidal areas (river water input) - visible as brown bands in the skeleton. This can be interpreted as a protective device against high iron concentrations.
Before iron is dosed, it is essential to perform ICP-MS analysis. ICP-OES is not sufficient to detect a deficiency. If iron is post-dosed, it should definitely be in the divalent form, as not all organisms can form siderophores and capture the trivalent iron.
Iron sources:
Sea salt, iron additives, feed, plankton, iron-based phosphate adsorbers, lime reactors, defective pumps, steel parts such as screws. In nature, also from river water.
Iron sinks:
Zeolites, water changes, precipitation processes.
Recommendation for seawater aquaria: 1...3 µg/l
Natural seawater concentration: 0.1..62 µg/l (at 35 PSU)
Christine Ferrier-Pagès, Vanessa SChoelzke, Jean Jaubert, Len Muscatine, Ove Hoegh-Guldberg 2001: Response of a scleractinian coral, Stylophora pistillata, to iron and nitrate enrichment.
B. E. Brown, A. W. Tudhope, M. D. A. Le Tissier & T. P. Scoffin 1991: A novel mechanism for iron incorporation into coral skeletons.
B. Entsch, R. G. Sim & B. G. Hatcher 1983: Indications from photosynthetic components that iron is a limiting nutrient in primary producers on coral reefs.
J. Malcolm Shick, Katrina Iglic, Mark L. Wells, Charles G. Trick, Jason Doyle, Walter C. Dunlap 2011: Responses to iron limitation in two colonies of Stylophora pistillata exposed to high temperature: Implications for coral bleaching.
Justin Leigh-Smith, Amanda Reichelt-Brushett, Andrew L. Rose 2017: The characterization of iron (III) in seawater and related toxicity to early life stages of scleractinian corals.
Nicholas E. Pingitore Jr, Arturo Iglesisas, Allison Bruce, Farrel Lytle, Gerard M. Wellington 2002: Valences of iron and copper in coral skeleton: X-ray absorption spectroscopy analysis.
Hannah G. Reich, Irene B. Rodriguez, Todd C. LaJeunesse, Tung-Yuan Ho 2020: Endosymbiotic dinoflagellates pump iron: differences in iron and other trace metal needs among the Symbiodiniaceae.
K. Vijayavel, C.A. Downs, G.K. Ostrander, R.H. Richmond 2012: Oxidative DNA damage induced by iron chloride in the larvae of the lace coral Pocillopora damicornis.
A.D. Harland, B.E. Brown 1989: Metal tolerance in the scleractinian coral Porites lutea.
Nils Rädecker, Claudia Pogoreutz, Maren Ziegler, Ananya Ashok, Marcelle M. Barreto, Veronica Chaidez, Carsten G. B. Grupstra, Yi Mei Ng, Gabriela Perna, Manuel Aranda, Christian R. Voolstra 2017: Assessing the effects of iron enrichment across holobiont compartments reveals reduced microbial nitrogen fixation in the Red Sea coral Pocillopora verrucosa.
Alice C. A. Blanckaert, Dario Omanović, Maoz Fine, Renaud Grover, Christine Ferrier-Pagès 2022: Desert dust deposition supplies essential bioelements to Red Sea corals.
Maria Eliza Nagel-Hassemer, Lucila Adriani Coral, Bruno Segall Pizzolatti, Luciano Vitali, Maria Eliza Hagel-Hassemer, Flávio Rubens Lapolli, Maria Ángeles Lobo-Recio 2014: Adsorption behaviour of the zeolite, Controll M.F. 574 in removing iron and manganese from natural water.
Philip Jay Motta 1987: A quantitative analysis of ferric iron in butterflyfish teeth (Chaetodontidae, Perciformes) and the relationship to feeding ecology.